Xfect RNA Transfection Reagent
Xfect RNA Transfection Reagent
Xfect RNA Transfection Reagent
Xfect RNA Transfection Reagent is a complete system for highly efficient transfection of mammalian cells with all types of RNA, including messenger RNA, siRNA, single guide RNA (sgRNA), and microRNA. The protocol is simple, requires no optimization, and transfections can be carried out entirely in the presence of serum. While most transfection reagents can be harmful to your cells, the Xfect RNA Transfection Reagent has very low cytotoxicity.
Overview
- Single reagent for transfection with messenger RNA (mRNA), single guide RNA (sgRNA), microRNA (miRNA), and siRNA
- High transfection efficiency and very low cytotoxicity in cell lines and primary cells
- Simple, serum-compatible protocol
- Complete system that includes all components necessary for transfection
- Transfecting mRNA results in faster expression of your protein
- This reagent cannot deliver DNA, but can be combined with Xfect Transfection Reagent for efficient cotransfection of mammalian cells with both DNA and RNA (protocol described in the user manual)
Applications
- Knockdown of gene expression using siRNA
- Transient transfection with mRNA transcripts
- Knockout of gene expression using CRISPR/Cas9-mediated gene editing
Efficient transfection of microRNA using the Xfect RNA Transfection Reagent
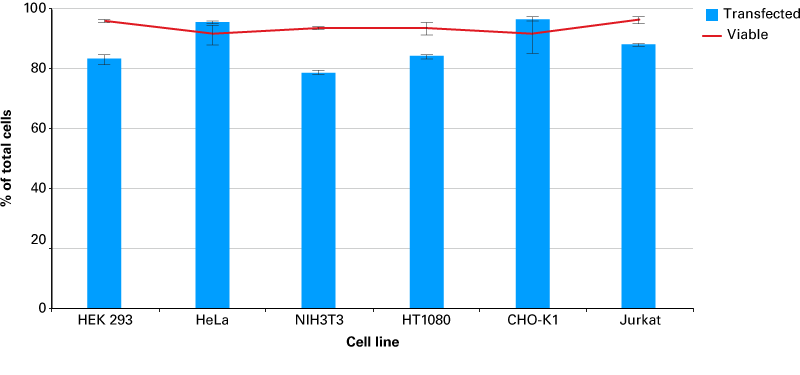
Efficient transfection of microRNA using the Xfect RNA Transfection Reagent. A FAM-labeled microRNA mimic was transfected into six popular cell lines using the the Xfect RNA Transfection Reagent according to the protocol. 24 hr post transfection, cells were harvested and transfection efficiency was determined by flow cytometry. Cell viability was also measured using Trypan Blue staining. Data represents the average of three independent transfections for each cell line.
Successful knockdown in primary cells and cell lines treated with siRNA
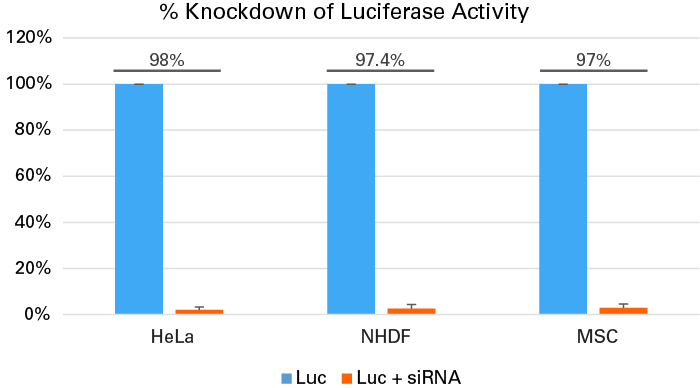
Successful knockdown in primary cells and cell lines treated with siRNA. HeLa cells (2.0 x 105), human dermal fibroblasts (NHDF) cells (6.0 x 104), and mesenchymal stem cells (MSCs) (5.0 x 104) were plated in 12-well plates and transfected with 50 pmol of siRNA against luciferase using Xfect RNA Transfection Reagent. All three cell types were also transfected with 1 µg of pCMV-Luc using the Xfect Transfection Reagent. Luciferase assays were performed 48 hours post-transfection. For control samples, cells were transfected with pCMV-Luc but without the siRNA. We observed a dramatic (>95%) decrease in luciferase activity in all the cells treated with siRNA.
Expression of green fluorescent protein (GFP) from transfection with mRNA into primary cells and cell lines
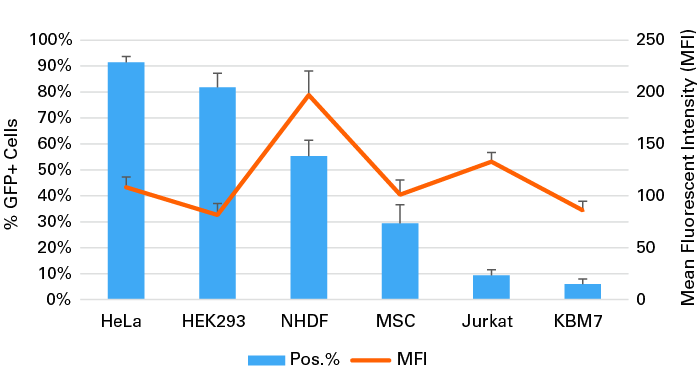
Expression of green fluorescent protein (GFP) from transfection with mRNA into primary cells and cell lines. HeLa cells (2.0 x 105), HEK 293 cells (1.5 x 105), human dermal fibroblasts (NHDF) cells (6.0 x 104), mesenchymal stem cells (MSCs) (5.0 x 104), Jurkat cells (3.0 x 105), and KBM7 cells (3.0 x 105) were plated in 12-well plates and transfected with 1 µg of mRNA encoding GFP with 5 µl of Xfect RNA Transfection Reagent. 20 hours later, cells were analyzed by flow cytometry and the % GFP-positive cells and the mean fluorescence intensity (MFI) were determined.
GFP is expressed in HeLa cells, NHDF cells, and MSCs
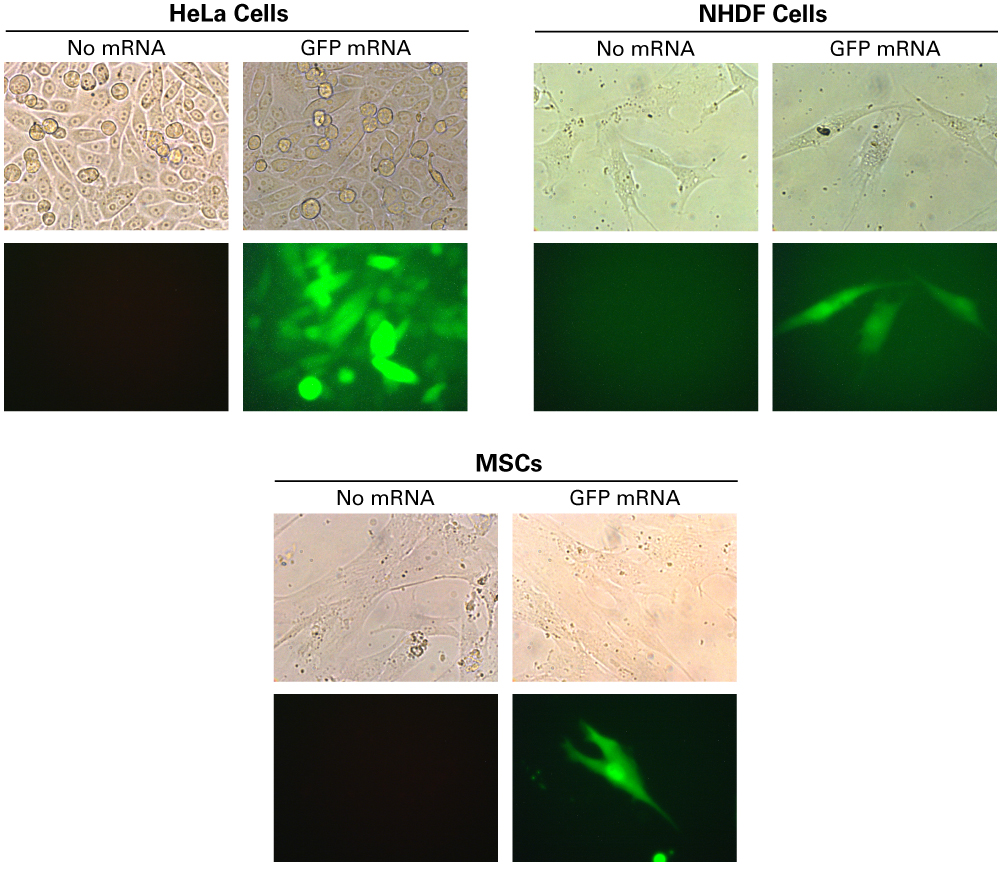
GFP is expressed in HeLa cells, NHDF cells, and MSCs. HeLa cells (2.0 x 105), human dermal fibroblasts (NHDF) cells (6.0 x 104), and mesenchymal stem cells (MSCs) (5.0 x 104) were plated in 12-well plates and treated with 1 µg of mRNA encoding GFP with 5 µl of Xfect RNA Transfection Reagent. 20 hours later, cells were imaged using an epifluorescent microscope. Images of HeLa cells were taken at 20X magnification. Pictures of NHDF cells and MSCs were taken at 40X.
Functional knockout of the endogenous CD81 gene by sgRNA transfection
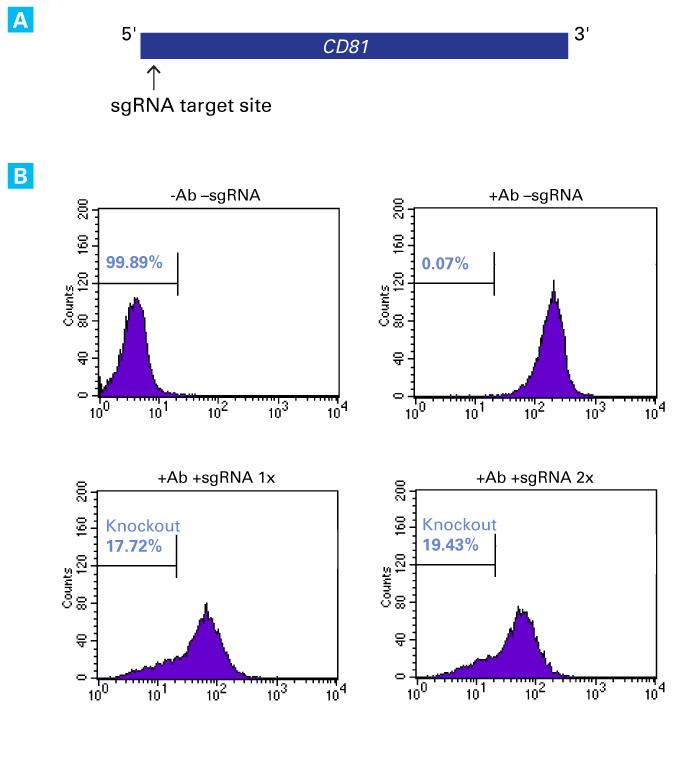
Functional knockout of the endogenous CD81 gene by sgRNA transfection. Panel A. sgRNA targeting the 5’ end of the antisense strand of CD81 was synthesized using the Guide-it sgRNA In Vitro Transcription Kit. Panel B. An HT1080 cell line (2.0 x 105 cells) stably expressing Cas9 (HT1080-Cas9) was transfected with 50 pmol of sgRNA targeting CD81, either once or twice (lower graph), using the Xfect RNA Transfection Reagent. Seven days later, cells were immunostained with a CD81 antibody (Ab) conjugated to a FITC fluorophore and analyzed by flow cytometry. The percentage of cells that did not bind CD81 was calculated. A control sample, comprised of HT1080-Cas9 cells, was analyzed by flow cytometry, either without (top, left graph) or with (top, right graph) the CD81 antibody. Both single and double transfection with sgRNA resulted in a substantial increase in cells that did not bind CD81, indicating successful CRISPR/Cas9-mediated knockout of CD81.
Knockout of the luciferase gene by sgRNA transfection
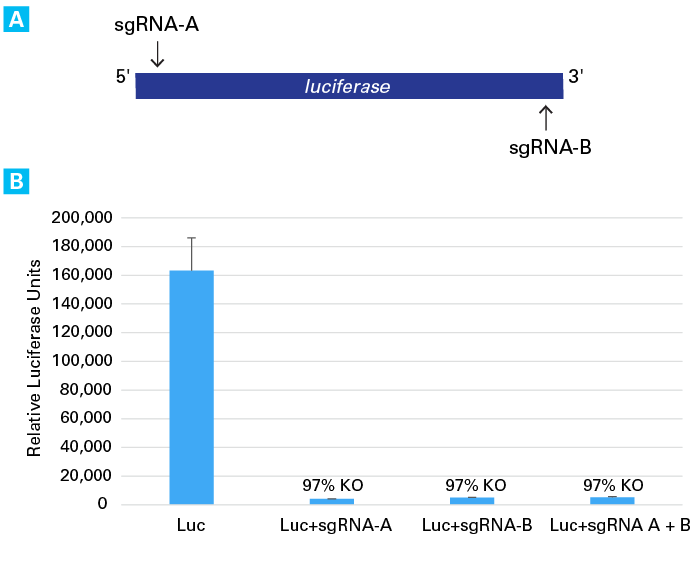
Knockout of the luciferase gene by sgRNA transfection. Panel A. Two sgRNAs (sgRNA-A and sgRNA-B) targeting sense and antisense strands of luciferase were synthesized using the Guide-it sgRNA In Vitro Transcription Kit. Panel B. 3.0 x 105 HT1080 cells stably expressing Cas9 (HT1080-Cas9) were plated in 12-well plates and transfected with 1 µg of pCMV-Luc (using the Xfect Transfection Reagent) and 200 ng of either sgRNA-A, or sgRNA-B, or 100 ng each of both sgRNAs (using the Xfect RNA Transfection Reagent). 48 hours post-transfection, luciferase assays were performed. Robust knockout (>95%) of luciferase activity was observed with each sgRNA individually and in combination with each other.
The CRISPR/Cas9 system, a simple, RNA-programmable method to mediate genome editing in mammalian cells
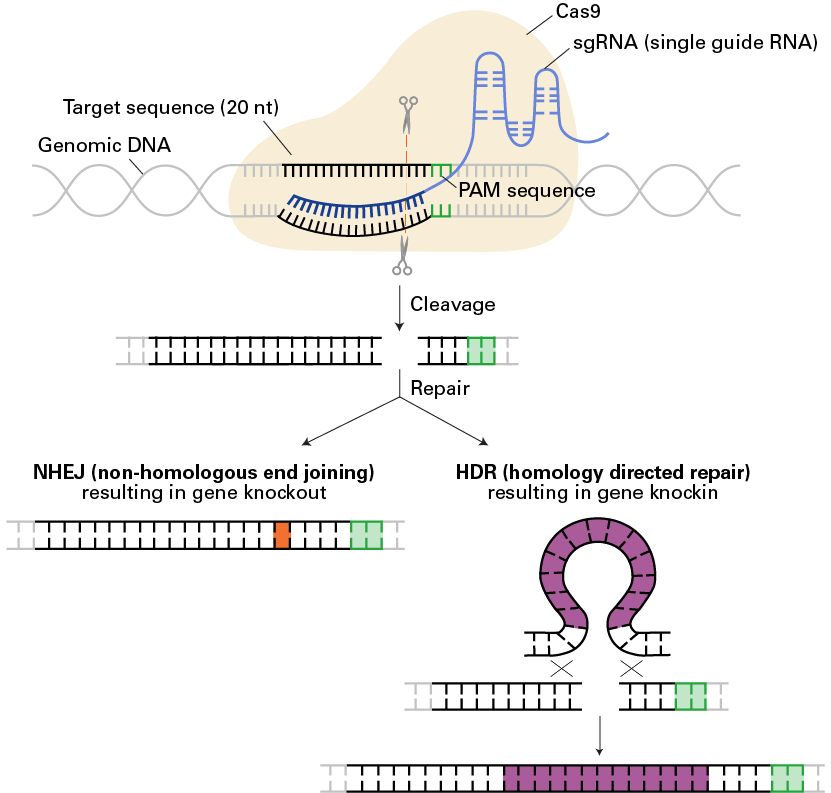
The CRISPR/Cas9 system, a simple, RNA-programmable method to mediate genome editing in mammalian cells. The CRISPR/Cas9 system relies on a single guide RNA (sgRNA) directing the Cas9 endonuclease to induce a double strand break at a specific target sequence three base-pairs upstream of a PAM sequence in genomic DNA. This DNA cleavage can be repaired in one of two ways: 1) nonhomologous end joining, (NHEJ) resulting in gene knockout due to error-prone repair (orange), or 2) homology-directed repair (HDR), resulting in gene knockin due to the presence of a homologous repair template (purple).
Xfect MicroRNA Transfection Reagent successfully transfects firefly luciferase (Fluc) messenger RNA into HeLa cells
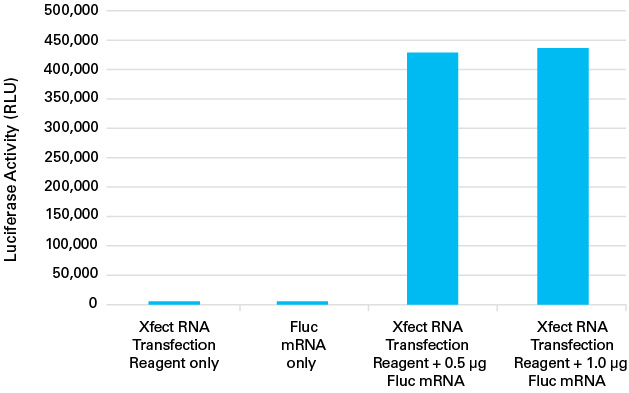
Xfect MicroRNA Transfection Reagent successfully transfects firefly luciferase (Fluc) messenger RNA into HeLa cells. Fluc messenger RNA (0.5 μg and 1.0 μg) was transfected into HeLa cells using Xfect MicroRNA Transfection Polymer according to the protocol described above. 24 hours after transfection, luciferase activity (Relative Light Units; RLU) was measured using a luciferase assay.