TransIT-X2 Dynamic Delivery System
TransIT-X2 Dynamic Delivery System
A dynamic transfection system for the delivery of plasmid DNA, siRNA/miRNA and CRISPR/Cas9 components
A dynamic transfection system for the delivery of plasmid DNA, siRNA/miRNA and CRISPR/Cas9 components
Move your research forward with a versatile transfection reagent that is the solution for hard-to-transfection and common cell types. Proven and reliable, TransIT-X2® allows you to avoid guesswork and get it right the first time.
- Peak performance in a broad range of cell types
- Suitable for multiple applications
- Efficient delivery of plasmid DNA, siRNA/miRNA and CRISPR/Cas9 components
- Learn more about using TransIT-X2® for CRISPR/Cas9 genome editing
Additional Resources
- TransIT-X2® Cell Type Specific Protocol Optimization Card (PDF)
- Poster: Optimization of DNA, RNA and RNP Delivery Methods for Efficient CRISPR/Cas9 Mediated Cell Engineering (PDF)
Technical Product Literature
Full Protocols
TransIT-X2® Full Transfection Protocol (PDF)
Additional Protocols
CRISPR pDNA/gRNA Delivery Protocol (PDF)
CRISPR RNP + ssODN Delivery Protocol (PDF)
TransIT-X2® for CRISPR Plasmid Delivery Protocol (PDF)
TransIT-X2® for CRISPR RNP + ssODN Delivery (PDF)
TransIT-X2® for CRISPR RNP Delivery (PDF)
Quick Reference Protocols
TransIT-X2® Quick Ref Protocol (PDF)
SDS
Optimization Protocols
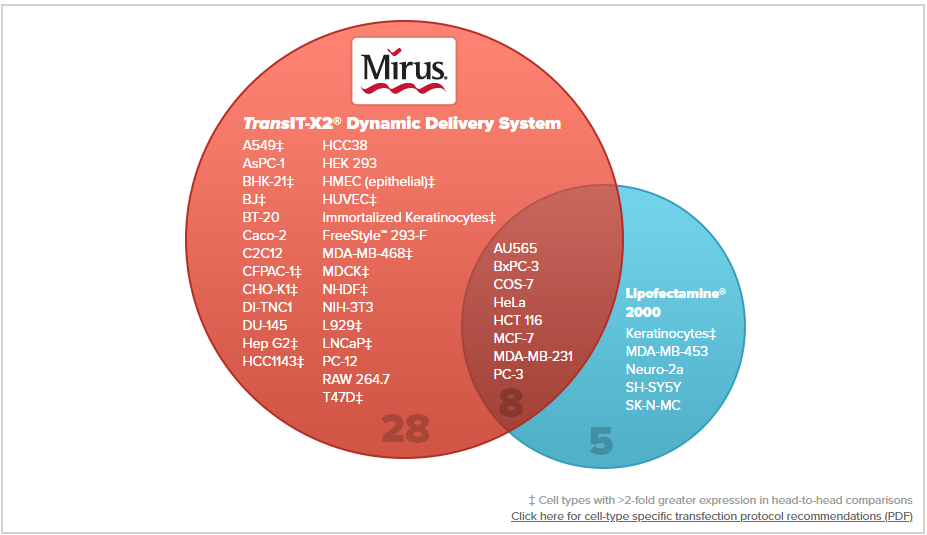
TransIT-X2® System Enables Superior Gene Expression in a Variety of Cell Types
TransIT-X2® Transfection Reagent Enables Superior Transfection in a Variety of Cell Types. TransIT-X2® Dynamic Delivery System and Lipofectamine® 2000 Transfection Reagent were used to transfect plasmid DNA encoding luciferase into 41 different cell types at three reagent-to-DNA ratios. Luciferase expression was compared at 24 hours post-transfection using a standard luciferase assay. Head-to-head comparisons at optimized ratios illustrate superior or equal luciferase expression using TransIT-X2® in 36 of 41 cell types; 17 cell types that had expression levels 2-fold higher are denoted with ‡.
Figures and Data

TransIT-X2® Dynamic Delivery System Outperforms Lipofectamine® Reagents. A549 (A) or MDCK (B) cells were transfected with luciferase encoding plasmid DNA using either TransIT-X2® (Mirus Bio), Lipofectamine® 2000 (Thermo Fisher Scientific) or Lipofectamine® 3000 (Thermo Fisher Scientific) for 24 hours at indicated reagent-to-DNA ratios or reagent-to-P3000™-to-DNA ratio. Transfection was measured by luciferase activity using a conventional assay. Cytotoxicity was assessed by quantifying the LDH released from the cytosol of damaged cells compared to cells alone. Higher transgene expression and lower cytotoxicity was observed in cells transfected with TransIT-X2® at optimal ratios compared to cells transfected with Lipofectamine® 2000 or Lipofectamine® 3000.

Visualization of High GFP Expression Using TransIT-X2® Dynamic Delivery System. TransIT-X2® Dynamic Delivery System was used to transfect plasmid DNA encoding EGFP into A549, CHO-K1, HepG2, LNCaP, MDCK, PC12, primary human mammary epithelial cells (HMEC) and normal human dermal fibroblasts (NHDF). Transfections were performed in 35 mm MatTek dishes using 4-8 µl of TransIT-X2® to deliver 2 µg of DNA. Images (32X) were captured at 48 hours post-transfection using a Zeiss Axiovert S100 inverted fluorescence microscope.

High GFP Transfection Efficiency in Multiple Cell Lines and Primary Cells Using TransIT-X2® Dynamic Delivery System. TransIT-X2® Dynamic Delivery System was used to transfect plasmid DNA encoding EGFP into A549, CHO-K1, Hep G2, MDCK, LNCaP, PC-12, primary human mammary epithelial cells (HMEC) and normal human dermal fibroblasts (NHDF). Transfections were performed in 96-well plates using 0.2-0.4 µl of TransIT-X2® to deliver 0.1 µg of DNA (2:1, 3:1 or 4:1 reagent: DNA ratio). Triplicate wells were assayed 48 hours post-transfection using guava easyCyte™ 5HT Flow Cytometer.

Functional Co-transfection of Plasmid DNA and siRNA Using the TransIT-X2® Dynamic Delivery System. TransIT-X2® Dynamic Delivery System was used to transfect plasmid Cy®5 labeled DNA encoding nuclear YFP and Cy®3 labeled siRNA into HeLa cells. Transfection was performed in 6-well plates with Poly-L-Lysine (PLL) coated coverslips using 4 µl of TransIT-X2® to deliver 2 µg of DNA and 25 nM siRNA (2:1 reagent:DNA ratio). Actin cytoskeleton was stained using Alexa Fluor® 350 Phalloidin. Images (63X) were captured at 24 hours post-transfection using a Nikon A1R confocal microscope. Image key: yellow (nuclear YFP), blue (Cy®5 labeled DNA), red (Cy®3 labeled siRNA), green (actin cytoskeleton).

TransIT-X2® Dynamic Delivery System Achieves Higher Knockdown than Lipofectamine® 2000. TransIT-X2® Dynamic Delivery System and Lipofectamine® 2000 Transfection Reagent were used to transfect siRNA targeting endogenous proteins – GAPDH and AHA1 or to deliver a non-targeting siRNA control in normal human dermal fibroblasts (NHDF). Cells were transfected in a 6-well plate using 4 µl of TransIT-X2® or 6 µl of Lipofectamine® 2000 and 25 nM siRNA according to each manufacturer's protocol. The amount of GAPDH or AHA1 mRNA was measured relative to 18s rRNA levels using qRT-PCR and then normalized to the mRNA levels of the non-targeting control, 48 hours post-transfection. Error bars represent the standard deviation of triplicate wells.

TransIT-X2® Outperforms Lipofectamine® for RNP Delivery. Ribonucleoprotein (RNP) complexes composed of PPIB (cyclophilin B) targeting 2-part gRNA (IDT) and Cas9 protein (PNA Bio) were delivered into HEK293T/17 and U2OS cells using TransIT-X2® Dynamic Delivery System (1 µl/well, Mirus Bio) or Lipofectamine® CRISPRMAX™ (1.5 µl/well and 1 µl/well of Lipofectamine® Cas9 Plus™ Reagent, ThermoFisher) or Lipofectamine® RNAiMAX (1.5 µl/well, ThermoFisher) or Lipofectamine® 3000 (1.5 µl/well and 1 µl/well of P3000™ Reagent, ThermoFisher) in a 24-well format according to the manufacturers’ protocol. Varying levels of gRNA (6 nM or 12 nM) were tested with 6 nM Cas9 protein (PNA Bio). A T7E1 mismatch detection assay was used to measure cleavage efficiency at 48 hours post-transfection.