RetroNectin GMP grade
RetroNectin GMP grade
RetroNectin GMP grade
T202, RetroNectin GMP grade, is manufactured and tested in accordance with the following guidelines, "Standards for Manufacturing Control and Quality Control, etc. of Investigational Products (Investigational products GMP)" notified by the Ministry of Health and Welfare Japan ("PFSB Notification No. 0709002, July 9, 2008)" (PFSB: Pharmaceutical and Food Safety Bureau).
RetroNectin reagent is a 63 kD fragment of recombinant human fibronectin fragment (also referred to as rFN-CH-296) that enhances the efficiency of lentiviral- and retroviral-mediated gene transduction. This is particularly important for hematopoietic cells and other hard-to-transfect cell types. Enhanced transduction is thought to result from co-localization of virus particles and target cells. This is accomplished by direct binding of viral particles to sequences in the heparin-binding domain and interaction of target-cell integrins with two other domains in rFN-CH-296. RetroNectin GMP grade is manufactured as a quality-assured product, according to relevant guidelines for Good Manufacturing Practice (GMP). This product can be used for ex vivo clinical applications. Researchers are free to use this product for development of research-based clinical trials. Proper regulatory approval is required before using the GMP-grade product in research-based clinical trials since these trials may require special considerations or more stringent manufacturing standards. This product is not intended for human in vivo applications. It is the end user’s responsibility to ensure that the final product meets the requirements of the application for which it is to be used. Takara Bio Inc., has submitted a Drug Master File (DMF application number 18898) to the Food and Drug Administration for RetroNectin GMP grade.
Overview
- Enhanced gene transfer efficiency for retroviral or lentiviral transduction of suspension cells, hematopoietic stem cells, and other hard-to-infect cell types
- Less toxicity than other commonly used transduction enhancers, including polybrene and protamine
- Fully recombinant peptide
- The efficiency of T cell (CD8+) expansion can be significantly increased in the presence of RetroNectin reagent
- T202, RetroNectin® GMP grade, is manufactured and tested in accordance with the following guidelines, "Standards for Manufacturing Control and Quality Control, etc. of Investigational Products (Investigational products GMP)" notified by the Ministry of Health and Welfare Japan ("PFSB Notification No. 0709002, July 9, 2008)" (PFSB: Pharmaceutical and Food Safety Bureau)
- Takara Bio Inc., has submitted a Drug Master File (DMF application number 18898) to the Food and Drug Administration for RetroNectin GMP grade.
Applications
- Enhanced retroviral or lentiviral mediated transduction of cells that express VLA-4 or VLA-5 (including many hematopoietic cell types)
- In vitro T-cell expansion of CD8+ cells
- RetroNectin reagent has been used in over 40 clinical trials
- GMP-grade RetroNectin (Cat. # T202): Manufactured as a quality-assured product, according to relevant guidelines for Good Manufacturing Practice (GMP). This product can be used for ex vivo clinical applications.
Comparison of T cell expansion efficiencies for various commonly used protocols

Comparison of T cell expansion efficiencies for various commonly used protocols. Cells co-stimulated using RetroNectin and α-CD3 demonstrated higher-fold expansion as compared to other protocols (Panel A). In addition, the presence of RetroNectin promoted the production of more naïve-like T cells (CCR7+/CD45RA+ phenotype) that have been shown to have better in vivo anti-tumor activity (Panel B).
NOTE: This expansion method requires a license for commercial applications.
Comparison of retroviral transduction of a K562 cell line using transduction enhancers from different companies

Comparison of retroviral transduction of a K562 cell line using transduction enhancers from different companies. A K562 cell line was transduced with a retroviral vector in the presence of various transduction enhancers. RetroNectin yielded a far higher transduction efficiency relative to each of the other transduction enhancers, including Polybrene, protamine sulfate, and human fibronectin from three other companies.
Efficient transduction of human PBMCs using RetroNectin, as compared to Polybrene

Efficient transduction of human PBMCs using RetroNectin, as compared to Polybrene. Human PBMCs were transduced with varying dilutions of lentivirus encoding ZsGreen1 in the presence of RetroNectin or Polybrene, and the percentages of transduced CD8+ cells were measured by flow cytometry. Transduction efficiencies with Retronectin were significantly higher than those obtained using Polybrene, especially at low MOI (90X dilution).
T-cell expansion using RetroNectin reagent.
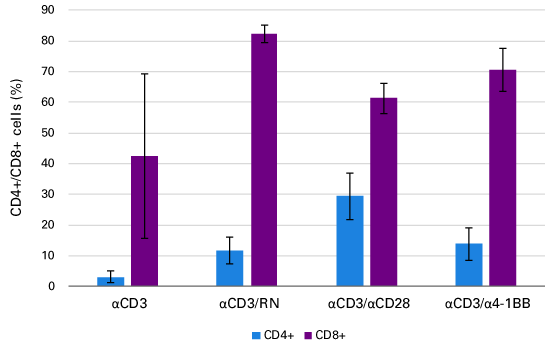
T-cell expansion using RetroNectin reagent. Peripheral blood T cells were stimulated and expanded in vitro using anti-CD3 (clone OKT3) and IL-2 alone or in combination with RetroNectin reagent (RN), anti-CD28, or anti-4-1BB. Stimulation with RetroNectin reagent yielded higher proportions of cytotoxic CD8+ T cells.


