Lenti-X Packaging Single Shots
Lenti-X Packaging Single Shots
Lenti-X packaging single shots are fourth-generation lentiviral packaging systems utilizing an extremely simple, consistent one-step method for producing high-titer lentivirus. Typically, titers of 107–108 IFU per ml can be expected for the VSV-G version.
No additional transfection reagent is needed because Lenti-X packaging single shots consist of prealiquoted, lyophilized, single tubes of Xfect Transfection Reagent premixed with an optimized formulation of Lenti-X packaging plasmids. High-titer virus is produced by simply reconstituting this mixture with your lentiviral vector of choice in sterile water and adding it to 293T cells, e.g., Lenti-X 293T Cells (Cat. # 632180), in a 10-cm dish.
Choose from three types of Lenti-X Packaging Single Shots optimized for different tropisms and integration properties:
- Lenti-X Packaging Single Shots (VSV-G) provide the highest titers (107–108 IFU per ml) and widest tropism
- Lenti-X Packaging Single Shots (Ecotropic) restrict tropism to cells of mouse and rat origin
- Lenti-X Packaging Single Shots (Integrase Deficient) produce VSV-G pseudotyped lentivirus that has a significantly reduced ability to integrate
The amount of reagent and vector in each tube is optimized for lentivirus production in a 10-cm dish. Transfections can be carried out entirely in the presence of serum. Use of tetracycline-free FBS is critical for achieving high titers with this technology.
Overview
- Fourth-generation lentiviral packaging system combines unparalleled convenience with high performance
- Simple protocol—Just add your lentiviral DNA, mix, and add to 293T cells
- No optimization or additional transfection reagent required
- Individual tubes contain lyophilized transfection reagents (Xfect polymer and buffer) and lentivirus packaging plasmids (five-component mix combined in an optimized ratio)
- The amount of reagent and vector in reaction is optimized for lentivirus production in a 10-cm dish
- Choice of three types of Lenti-X packaging single shots optimized for VSV-G pseudotyped, ecotropic, or integrase-deficient lentivirus
- Consistently high titers (107–108 IFU/ml)
Applications
- Lentiviral transduction of a broad range of hard-to-transfect cell types
- Easily create stable cell lines
- Create VSV-G pseudotyped, ecotropic, or integrase-deficient lentivirus
The Lenti-X Packaging Single Shots (VSV-G) protocol
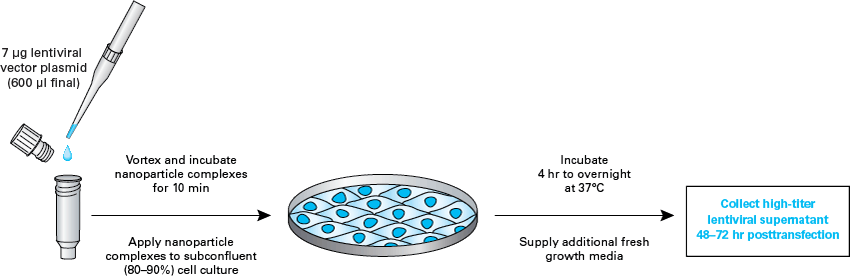
The Lenti-X Packaging Single Shots (VSV-G) protocol.
Consistent, high-efficiency transfections lead to high titers

Consistent, high-efficiency transfections lead to high titers. A lentiviral vector containing the ZsGreen1 gene was packaged according to the Lenti-X single shots protocol in four independent experiments. Briefly, 7 µg of pLVX-ZsGreen1 plasmid was added to each of four Lenti-X single shots; the tubes were vortexed for 20 sec and incubated at room temperature for 10 min. Then, the mixture was added to cultured Lenti-X 293T cells that were approximately 80% confluent. 48 hours after transfection, the cells were imaged by fluorescence microscopy (Panel A, top) and light microscopy (Panel A, bottom). After images were taken, the supernatant was harvested and used infect HT1080 cells for titer determination (Panel B, IFU/ml).
High-titer virus was produced regardless of the lentiviral vector backbone with Lenti-X packaging single shots

High-titer virus was produced regardless of the lentiviral vector backbone with Lenti-X packaging single shots. A CMV ZsGreen1 expression cassette was cloned into several lentiviral vector backbones. These vectors were then packaged into lentivirus using the Lenti-X packaging single shots following the provided protocol. Briefly, 7 µg of pLVX-ZsGreen1 plasmid was added to each of four Lenti-X single shots, and the tubes were vortexed for 20 sec and incubated at room temperature for 10 min. Then, the mixture was added to cultured Lenti-X 293T cells that were approximately 80% confluent. After 48 hours, titer was determined using several methods. To determine infectivity, the supernatant was harvested and used to infect HT1080 cells (Flow Cytometry). Harvested viral supernatants were also analyzed by RT-PCR to quantify viral genome copies (qRT-PCR, Lenti-X qRT-PCR Titration Kit), ELISA to measure p24 (p24 ELISA, Lenti-X p24 Rapid Titer Kit), and by a rapid lentiviral detection method (Lenti-X GoStix).
A comparison of fourth- and third-generation lentiviral packaging systems

A comparison of fourth- and third-generation lentiviral packaging systems . Our Lenti-X Packaging single shots utilize a packaging system that consists of five separate components (Panel A), mixed in proprietary proportions for optimized packaging activity. The separation of the gag, pol, and env genes effectively reduces the incidence of RCL (Wu et al., 2000). High levels of expression of essential viral components are driven by the Tet-Off and Tat transactivators, which induce a cascade of expression that results in high titers of lentivirus. The pol gene is fused to vpr to ensure transport of the reverse transcriptase/integrase protein into the recombinant lentiviral particle. Not all vector elements are shown. Other 3rd generation lentiviral packaging systems (Panel B) generate lower titers, do not contain separate gag and pol sequences, and do not use a transactivation cascade mechanism.


