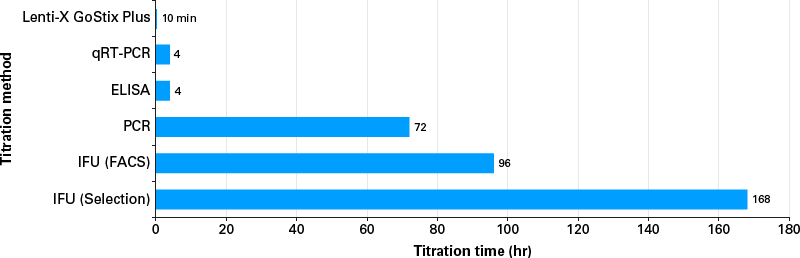
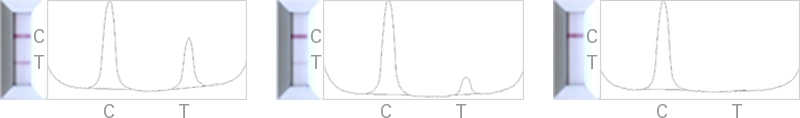
Lenti-X GoStix Plus provide a rapid, simple, and effective method for instantly quantifying lentivirus in packaging cell supernatants. The test involves applying 20 µl of supernatant to a GoStix cassette and waiting 10 minutes for the appearance of test and control bands that indicate the presence of lentiviral p24. The results on the cassette can then be analyzed using a free smartphone app, which quantifies lentivirus titer by comparing the intensities of the test and control bands.
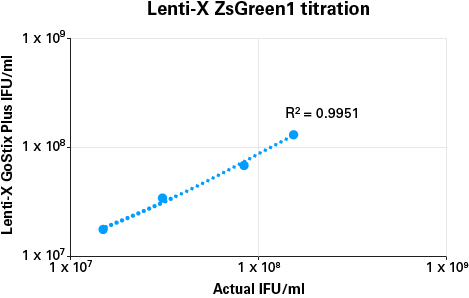
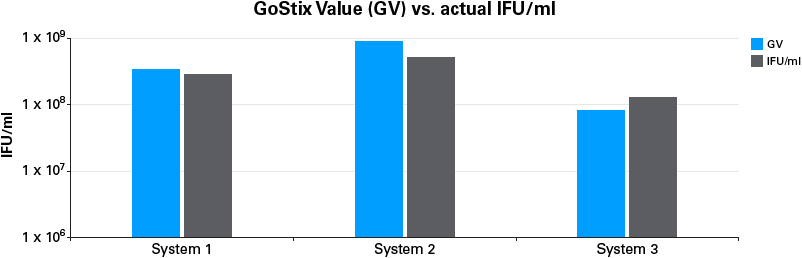
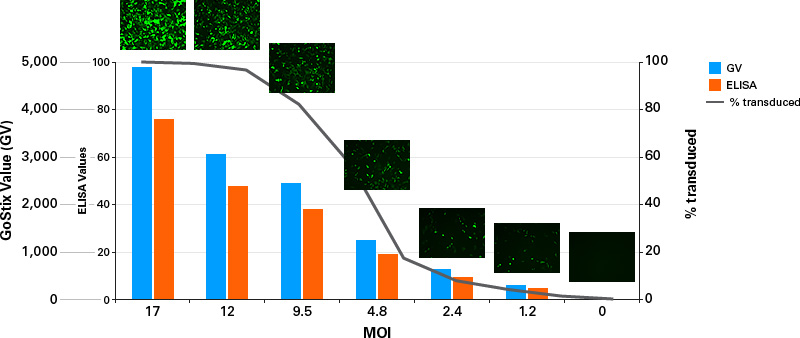
The quantitative output from the Lenti-X GoStix App (referred to as a GoStix Value, or GV) can be used to compare virus amounts between different preparations in a manner similar to, but far easier and quicker than, an ELISA, and allows for calculation of actual IFU/ml using a reference virus.
Overview
- Quantify lentivirus titer in 10 minutes using a simple smartphone app*
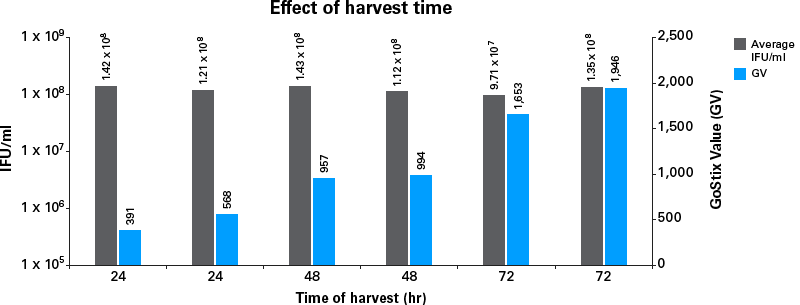
- Determine the optimal time to harvest lentiviral supernatants
- Achieve consistent transduction performance for your experiments
*The original Lenti-X GoStix (Cat # 631243 and 631244) cannot be used for lentivirus quantitation with the smartphone app and are being phased out. These items will no longer be available once inventories are depleted. If desired, Lenti-X GoStix Plus can be used for qualitative analyses in the same manner as the original Lenti-X GoStix.
Applications
- Confirm successful lentiviral packaging reactions
- Compare virus amounts between different preparations