Applications
- Extraction of viral RNA and DNA and microbial DNA from clinical samples
- Clinical testing applications
Features
- One kit for any common clinical sample type
- High sensitivity
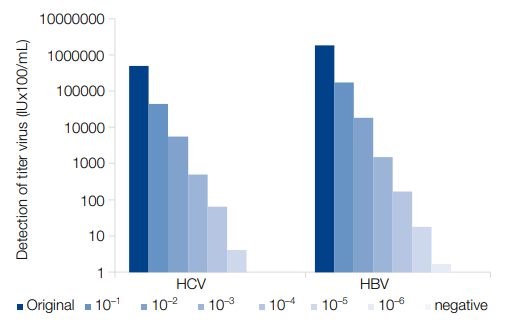
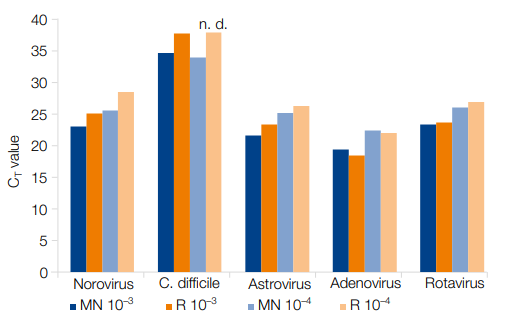
- Reliable nucleic acid isolation – suitable even for low viral titers
Specification
- Technology: Magnetic-bead technology
- Format: Highly reactive superparamagnetic beads
- Processing: Manual or automated
- Sample material: The prep is fully scalable. A convenient volume, especially for 96-well processing would be: < 200 µL whole blood, serum, plasma, < 25 mg tissue (e.g., ear notches), < 200 µL feces, < 200 µL swab wash solution
- Maximum amount of starting material in purification procedure: 200 µL liquid/homogenized sample
- Fragment size: 300 bp–approx. 50 kbp
- Elution volume: 50–100 µL
- Preparation time: 40–120 min/96 preps
- Binding capacity: 0.4 μg/μL beads