No matter your end goal—be it protein expression, high-throughput screening, cell engineering, etc.—taking the time to titrate your virus will increase the likelihood of a successful transduction. Knowing the number of particles, or infectious units, in your viral prep is important because it largely determines the transduction efficiency (i.e., how many viruses get into a cell), and thus the number of integrated gene copies in the transduced target cell. It is, therefore, worth taking time to understand the different measurement methods and how they relate to one another. Here’s a crash course on lentiviral titration to get your experiments started on the right foot.
Lentiviral production basics
There are two common measurement types: physical, which determine particle concentration by measuring corresponding nucleic acid or protein amounts, or functional, which count particles capable of productive transduction (IFU/ml). To understand what is being measured in these methods, let’s consider a lentiviral production workflow (Figure 1) to see what is being produced in a typical prep.
Recombinant lentiviruses are produced by cloning a sequence of interest into a lentiviral backbone containing all the necessary cis-acting elements required for virus function (Figure 1, Panel B). The resulting transfer vector is then mixed with a plasmid-based packaging mix that provides all the viral proteins required for virus particle production within a packaging cell. The transfer and packaging vectors are then combined with a transfection reagent (lipid or polymer), and the mixture is used to transfect a producer cell line, typically 293T-based cells, that can efficiently express the transfected constructs.

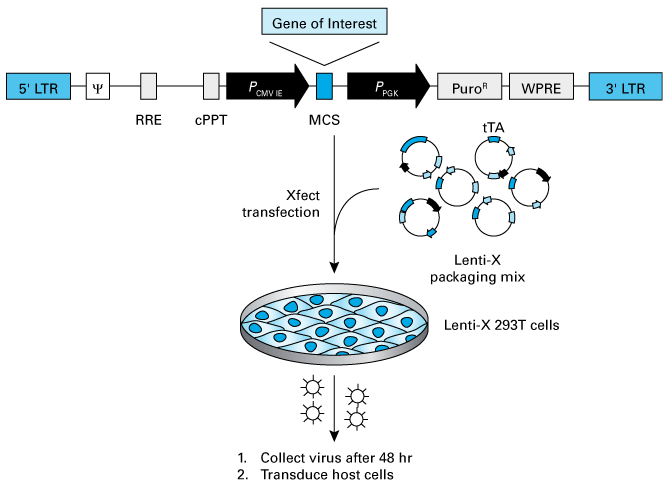

Figure 1. Example lentiviral production workflow. Panel A. Workflow for Takara Bio’s fourth-generation lentiviral packaging system. Panel B. Cis-acting elements in Takara Bio lentiviral vectors. Elements are derived from HIV-1.
The result is the release of viral particles into the supernatant. This output (Figure 2) includes fully complete and infectious particles, noninfectious particles, and free capsid proteins (i.e., p24). Physical titration measurements—qRT-PCR, p24 ELISAs, and Lenti-X GoStix Plus—are relatively straightforward to perform, but count both functional and nonfunctional virus particles (directly and/or via analysis of viral components released into supernatant). In contrast, functional titration methods specifically quantify particles capable of transducing target cells (i.e., infectious units or IFU), but are lengthier and more laborious.
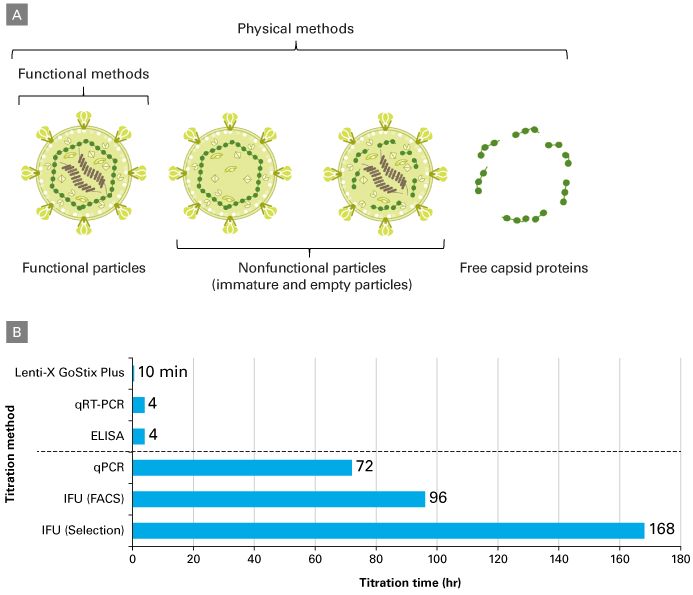
Figure 2. Types of titration methods. Panel A. Products present in lentiviral packaging supernatants. Physical methods can involve the measurement of both functional and nonfunctional particles, as well as free capsid proteins (e.g., p24) released in the supernatant. Functional titration methods specifically involve the measurement of functional viral particles. Panel B. Standard titration method timelines. Lenti‑X GoStix Plus: a lateral flow-based method for the detection of lentiviral p24 in supernatants. qRT-PCR: quantification of viral RNA genomes by qRT-PCR. ELISA: measurement of the amount of p24 capsid protein. qPCR: detection of integrated DNA proviruses by qPCR. IFU (FACS): determination of the percentage of infected cells via FACS analysis of RFP/GFP positive cells. IFU (Selection): infectious units determined by quantifying the number of drug-resistant colonies in transduced populations.
A key aspect of viral titration is that when viral production methods (Figure 3) are applied consistently, the ratios between analytes measured using physical or functional methods remain constant. What does this mean for you? In a nutshell, to quantitatively compare one prep to another using a physical titration method, all the production and postproduction processing steps must be performed the same way, and with the same reagents.

Figure 3. Influence of production methods on relationships between physical and functional measurements.
Let’s get physical
The great news is that while physical titration methods may not quantify functional particles directly, they provide rapid, reliable alternatives to functional titration methods once a relationship between the two measurements has been established. For example, let’s examine how a Lenti-X GoStix Plus assay measurement can be converted to an IFU/ml value with a little bit of math and a reference titer. Don’t run away; we promise it will be simple!
When you scan a GoStix cassette with the accompanying app, the app’s software uses densitometry to quantitate and compare band intensities on the cassette and then consults a lot-specific standard curve loaded onto your device to determine the corresponding quantity of p24 in the sample (ng/ml p24). To convert this GoStix measurement into a functional titer, a reference virus prepared using the exact conditions as your typical prep must first be measured using both a GoStix cassette and a functional titration method to determine the number of positively transduced cells per ml of supernatant from the reference virus prep (IFU/ml). Once both numbers are obtained, the ratio of IFU/ml to ng/ml p24 can be calculated. This value can then be used to convert any future measurements of ng/ml p24 to corresponding IFU/ml if the conditions and components are kept the same.

From titration to assays in a snap
Lentiviral titration is a critical aspect of viral transduction workflows because it ensures that preps are applied in sufficient quantities to obtain desired transduction efficiencies. However, traditional physical (e.g., qRT-PCR and ELISAs) and functional titration assays can be long and cumbersome, lengthening the time until you can get to your downstream assays. Since ratios of titration values obtained using physical and functional methods should be constant for a given lentiviral production workflow, this ratio can be used to convert measurements from a quick-and-easy physical titration method to a useful functional value. Now, you are a titration expert!
Your research time is valuable, so we’ve developed the 10-min Lenti-X GoStix Plus assay to provide a more rapid alternative to other physical titration methods such as qPCR and ELISA. Just apply your sample, wait ten minutes, and take a photo using the app. It’s that easy!

