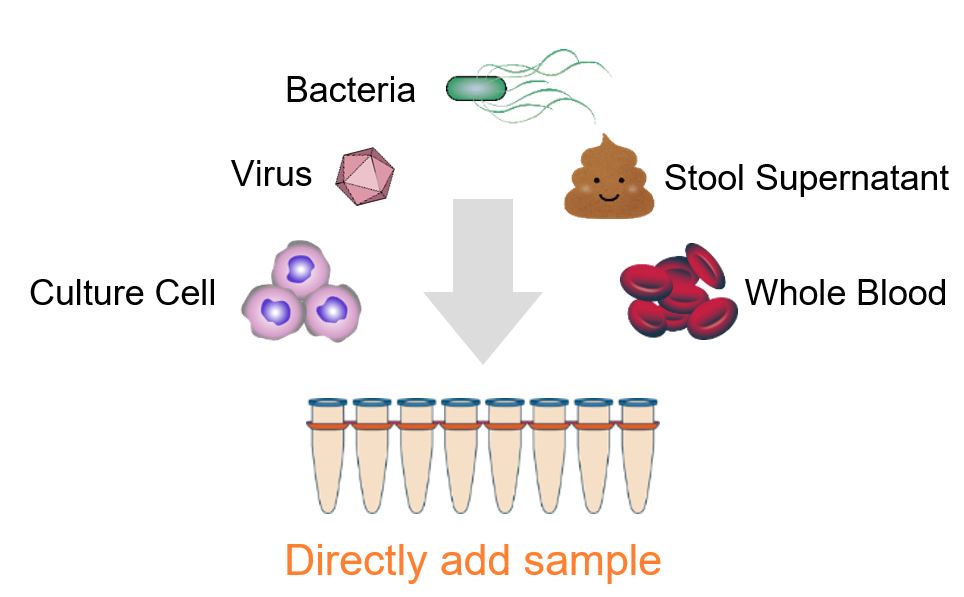
Detection of inactivated influenza A virus H1N1 with PrimeDirect™ Probe RT-qPCR Mix
Inactivated influenza A virus was spiked into oropharyngeal swab samples, nasopharyngeal swab samples and sputum samples at the concentration of 2 x 100-102 copies/µl, and the detection was performed for each sample type with PrimeDirect™ Probe RT-qPCR Mix. Results were generated by Takara Bio.
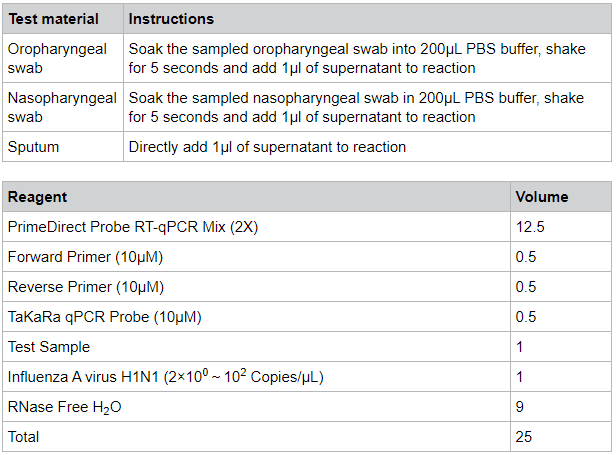
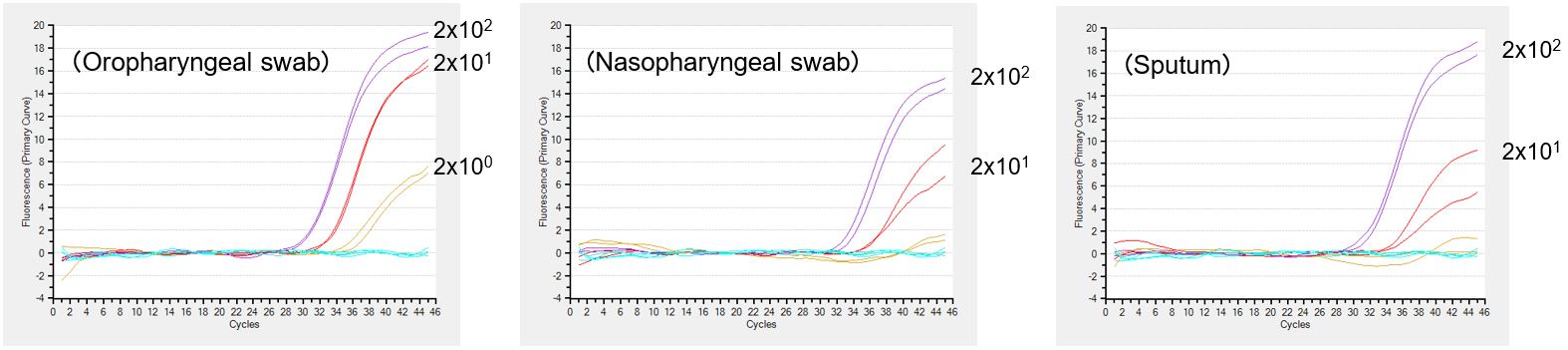
PrimeDirect™ Probe RT-qPCR Mix enabled the detection of viral RNA at 2 copies/µL from oropharyngeal swab samples. The detection sensitivity from nasopharyngeal swab samples and sputum samples was 20 copies/μL. This product is for research use only. It is not intended for therapeutic or diagnostic procedures.
Current Takara Bio one-step RT-qPCR kits being used for COVID-19 detection
Takara Bio has developed one-step probe RT-qPCR kits that are currently being used in the Chinese CDC and the Japanese NIID protocols for COVID-19 detection:
Chinese CDC Protocol |
Japanese National Institute for Infectious Diseases (NIID) Protocol |
Featured Product: |
Featured Product: |
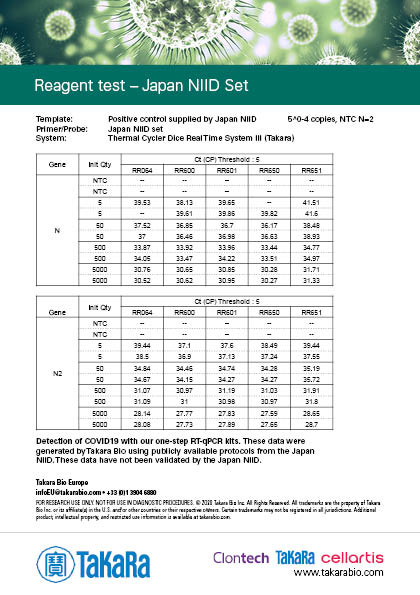
Compatibility with primer/probe sets from published COVID-19 detection protocols
|
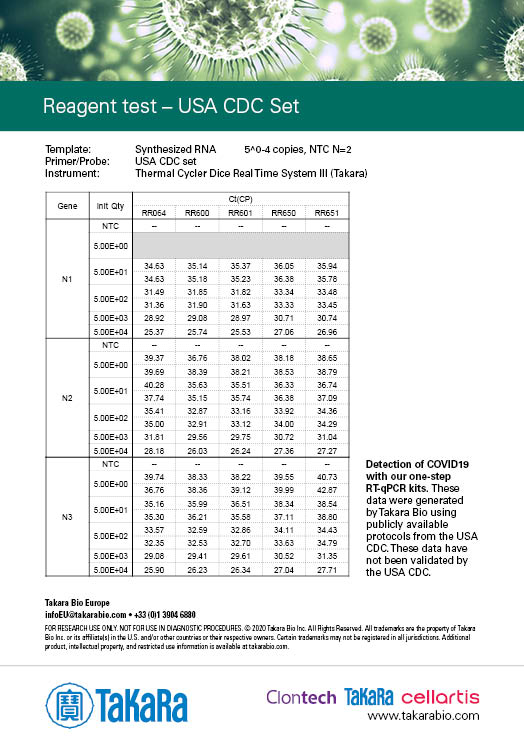
USA CDC Set
|
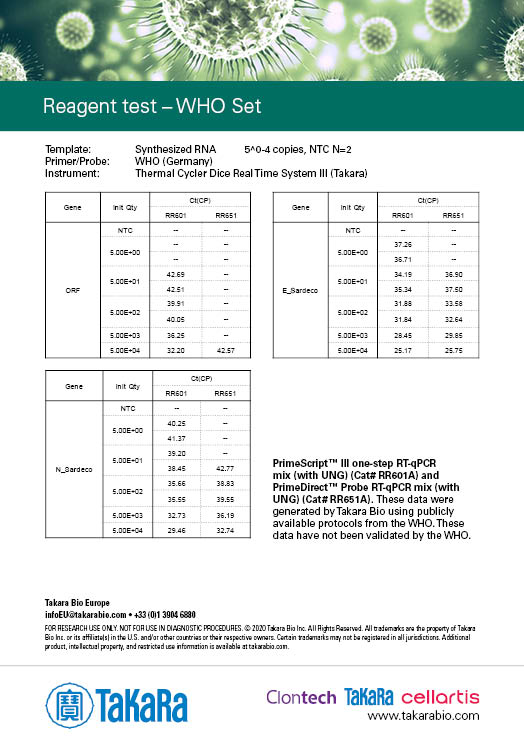
WHO Set
|
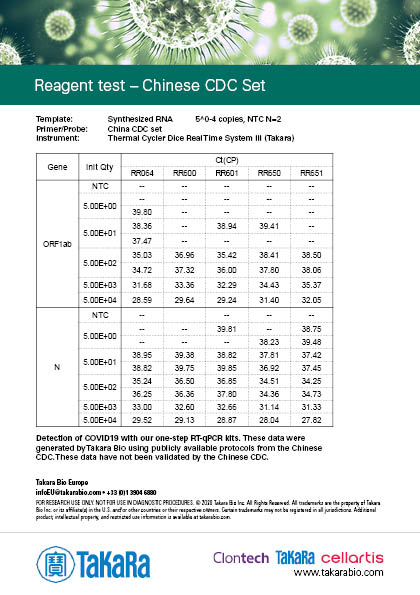
Chinese CDC Set
|
Japan NIID Set
|
The results were generated by Takara Bio by following the publicly available protocols. Our kits have not been validated by third parties and the data does not guarantee the performance in the protocol.
Sensitivity varies depending on the nature of the positive control. The positive control used in Japan NIID protocol was provided by the NIID (National Institute of Infectious disease), and the positive controls used in the other protocols were synthesized RNA prepared by Takara Bio.
Explore industry-leading products that can advance your COVID-19 research:
| Cat. # | Product | Sizes |
| RR650A/B | PrimeDirect™ Probe RT-qPCR Mix | 200 / 1,000 Rxns |
| RR651A/B | One Step PrimeScript™ RT-PCR Kit (Perfect Real Time) | 100 / 500 Rxns |
| RR600A/B | One Step PrimeScript™ III RT-PCR Kit | 200 / 1,000 Rxns |